Iron Mountain
The Geology and Mining of Iron Mountain
Geology
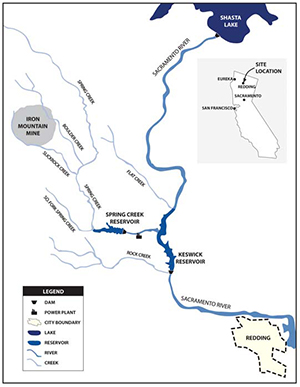
Iron Mountain is in Shasta County, California, approximately 14 km northwest of the town of Redding. In the 1860s, surveyor William Magee and settler Charles Camden noticed the striking red color of the rock outcrop on the south face of Iron Mountain and deduced the presence of "an immense iron deposit." In 1879, James Sallee discovered that the red rock -- known as gossan: rust-colored, oxidized iron ore -- contained silver as well as iron, and he, Magee, and Camden began mining the gossan and extracting the silver. In the mid-1890s, sulfide deposits within the mountain were discovered, and copper mining commenced. In subsequent years, Iron Mountain was also mined for gold, iron, zinc, and pyrite (iron sulfide); pyrite, a source of sulfur, was used to manufacture munitions and fertilizers and in petroleum refining. Mining exposed the minerals to water and air causing them to oxidize and release toxic constituents into the surrounding environment.
The massive sulfide deposits in the West Shasta mining district formed 350 to 400 million years ago in a marine environment. Geothermal hot springs on the sea floor expelled sulfur-rich hydrothermal fluids into the island arc setting. The brittle, fractured nature of the hydrothermally altered volcanic bedrock gives rise to hydrologic conditions dominated by fracture flow at Iron Mountain.
Mining Operations
The Iron Mountain mine is really a group of mines within Iron Mountain that includes Old mine, No. 8 mine, Confidence-Complex mine, Mattie mine, Richmond and Richmond Extension mines, Hornet mine, and Brick Flat mine. Nearly 100 years of mining activity at Iron Mountain provided an effective means for both water and air to reach the enormous sulfide deposits deep within the mountain, where water and oxygen reacted with the sulfide ores (mostly pyrite), to produce sulfuric acid and to dissolve the heavy metals in the ore. This is a classic recipe for acid mine drainage, which led one group of researchers to call Iron Mountain "a 'worst-case scenario' with respect to the formation of acid mine drainage." The Richmond Mine contains some of the most acid water in the world, with pH values having been measured as low as -3.6, combined metal concentrations as high as 200 g/liter, and sulfate concentrations as high as 760 g/liter. Most of the acid mine drainage comes from the oxidizing sulfides within the three largest sulfide ore bodies, the Brick Flat, Richmond, and Hornet deposits.
Stakeholders


News
July 26, 2018
Public lecture: Iron Mountain, California: An Extreme Acid Mine Drainage Environment, presented by Charles Alpers, USGS Research Chemist
Related Resources
USGS National Research Program: Chemical Modeling of Acid Waters, Iron Mountain, Shasta County, CA
U.S. Environmental Protection Agency: Pacific Southwest Superfund, Iron Mountain Mine

Contacts
Project Chief: Charles Alpers
Phone: 916.278.3134
Email: cnalpers@usgs.gov